Background: ITP is an acquired autoimmune disease characterized by transient or persistent thrombocytopenia. ITP substantially impairs pts' quality of life (QoL), including daily activities, social interactions, work and emotional wellbeing, with fatigue being the most common symptom reported by pts. Corticosteroids are the standard first-line treatment for ITP, while thrombopoietin receptor agonists (TPO-RAs), rituximab and fostamatinib are recommended for later lines; however, these treatments have limited sustained efficacy and may be associated with short- and long-term side effects that impact QoL.
Aim: To assess pt and physician (MD) perceptions of current ITP treatment and treatment goals using data from I-WISh 2.0.
Methods: I-WISh 2.0 was developed using findings from I-WISh 1.0 and surveyed 1018 pts with ITP aged >18 years and 431 MDs who managed ≥3 pts with ITP. I-WISh 2.0 was conducted from February to July 2022 in 15 countries. The surveys, developed by a steering committee (supported by Novartis) comprising expert ITP MDs and pt advocates, included questionnaires about prescribed ITP treatments, their impact on QoL and treatment satisfaction and goals.
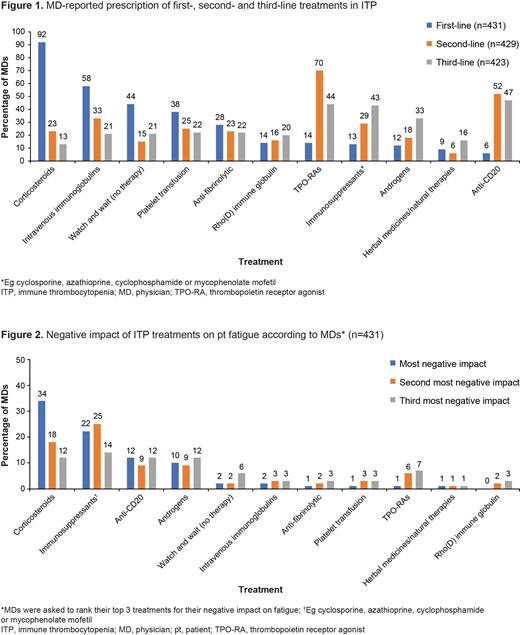
Results: Of 1018 pts (median age: 46 years), 67% were female and 48% reported ≥1 comorbidity. 97% (956/987) of pts had received ≥1 treatment for ITP and 12% (126/1018) had a splenectomy; 80% (811/1018) were receiving ITP treatment at survey completion and median time on most recent treatment was 30 weeks (n=608). Corticosteroids and TPO-RAs were the most common first- and second-line treatments prescribed by MDs, respectively (Figure 1); MDs estimated that 15% of their pts have opted against receiving ITP treatment (n=431). 73% of MDs (313/431) considered their pts' perception of medications when making treatment decisions.
Pts reported a significant burden due to their current ITP medication: 34% (202/597) experienced daily disruption, 37% (214/576) were bothered by needing to plan meals, and 22% (204/939) had difficulty keeping up with daily medications. 19% (172/897) of pts were dissatisfied with their treatment's platelet level control, and 18% (155/843) disagreed that their medication effectively controlled their symptoms. Pts worried about their treatment's short- (48%; 400/833) and long-term (62%; 527/844) side effects. 38% (162/431) of MDs were neutral toward current ITP treatment options and 9% (37/431) were dissatisfied. Of the 37 dissatisfied MDs, limited options (68%), inability to achieve deep/stable remission (54%), lack of efficacy (43%) and inability to reach treatment goals (43%) were the most common reasons.
32% (327/1016) and 13% (127/1016) of pts believed they were not or did not know if they were in sustained remission, respectively. A treatment offering a sustained remission or cure for ITP was an important goal for 82% (823/1007) of pts. Of treatment goals mentioned in their top 3, the most common were healthy blood counts (56%), improving QoL (44%) and increasing energy levels (43%) for pts (n=1018), and reducing spontaneous bleeds (79%), healthy blood counts (60%) and improving QoL (52%) for MDs (n=431). Limiting time on treatment would substantially affect the treatment preference of 66% (668/1009) of pts. 38% (294/775) of pts did not want to take their ITP treatment for the foreseeable future; however, 67% (680/1013) were concerned about relapse if they stopped.
Immunosuppression due to ITP treatment worried 414/787 (53%) pts, with 679/1007 (67%) considering it important when discussing treatment decisions. MDs considered corticosteroids and other immunosuppressants to have the most negative impact on fatigue (Figure 2), yet only 12% (52/431) strongly agreed they aimed to limit immunosuppressive effects.
Conclusions: Pts with ITP are dissatisfied with current treatment options because of a perceived lack of efficacy, the burden of short- and long-term side effects, and the need for daily, life-long administration. Additionally, some commonly prescribed treatments for ITP are associated with fatigue. Pts and MDs would like new ITP treatments to offer a sustained period of remission or cure, and pts wish to be able to discontinue them without fear of relapse. There is an unmet need for well-tolerated therapies with disease-modifying potential that can safely achieve these goals.
OffLabel Disclosure:
Bussel:Rigel: Consultancy; Sobi: Consultancy; UCB: Consultancy, Other: Data and safety monitoring board; argenx: Consultancy; Novartis: Consultancy; Amgen: Consultancy; AstraZeneca: Consultancy; Janssen: Consultancy. Cooper:Sobi: Honoraria; Rigel: Research Funding; Novartis: Honoraria, Research Funding; Sanofi: Honoraria. Ghanima:Sanofi: Consultancy, Honoraria; Bayer: Consultancy, Honoraria, Research Funding; Argenx: Consultancy, Honoraria; Kedrion: Consultancy; UCB: Consultancy, Honoraria; BMS: Honoraria, Research Funding; cellphire: Consultancy, Honoraria; Grifols: Consultancy, Honoraria; Sobi, Pfizer: Consultancy, Honoraria, Research Funding; alpine: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; hibio: Consultancy, Honoraria; Novartis: Consultancy, Honoraria. Provan:Amgen: Research Funding, Speakers Bureau; Novartis: Research Funding, Speakers Bureau; UCB: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; MedImmune: Consultancy; ONO: Consultancy; SOBI: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Argenx: Consultancy, Membership on an entity's Board of Directors or advisory committees; Rigel: Research Funding. Tomiyama:Kyowa Kirin: Honoraria; Novartis: Honoraria; Sysmex: Consultancy; Kissei Phamaceutical: Honoraria. Arnold:UpToDate: Patents & Royalties: Royalties; Sanofi: Consultancy; Rigel: Research Funding; Novartis: Consultancy; Rigel: Consultancy; Alpine Immune Sciences: Consultancy; Argenx: Consultancy; Novartis: Research Funding; Canadian Institutes of Health Research: Research Funding; Amgen: Consultancy; Medison: Consultancy; Sobi: Consultancy. Santoro:Novartis: Other: consulting, Speakers Bureau; Amgen: Other, Speakers Bureau. Zaja:Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Research Funding; Grifols: Consultancy, Honoraria, Research Funding; Sobi: Honoraria, Research Funding. Lovrencic:Swedish Orphan Biovitrum AB: Consultancy, Membership on an entity's Board of Directors or advisory committees; UCB: Membership on an entity's Board of Directors or advisory committees. Lahav:Novartis: Consultancy, Other. Winograd:Meddison: Consultancy, Other: grant; Novartis: Consultancy, Other: grant. Bailey:Adelphi Real World: Other: Tom Bailey is an employee of Adelphi Real World, which received funding from ADC Therapeutics to conduct the study.. Aziz:Novartis: Current Employment, Current equity holder in publicly-traded company. Abi Rached:Novartis: Current Employment.
Off-label use of rituximab is mentioned in the Background section, to explain that it is sometimes used to treat patients with ITP. Additionally, data are provided in the Results describing what first-, second- and third-line treatments physicians prescribe for ITP, as well as physician's opinions on which treatments have the most negative impact on ITP. Off-label treatments mentioned include anti-CD20, anti-fibrinolytics, androgens and immunosuppressants.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal